< Back
Intensifier Control

COMPACT AND COST EFFECTIVE
10-20 times more light efficiency in a compact and light weight form
HIGHLY CUSTOMISABLE
choose from a wide variety of high sensitivity image intensifiers to match the needs of your application
SINGLE-PHOTON SENSITIVE
high sensitivity supplemented with an acquisition speed up to 160 fps.
Description
The TRiCAM is a compact intensified low-light imaging camera. It is designed for scientific and industrial applications that require low-light imaging. With built-in signal generators, the TRiCAM is capable of ultra-short exposures through fast gating and therefore suitable for time-resolved imaging.
Applications
Laser Induced Fluorescence (LIF)
Time-gated luminescence
Bio- and chemiluminescence imaging
Plasma physics
Single Photon imaging
Particle Image Velocimetry (PIV)
Solar PV and LED characterization
Combustion research
Single-molecule imaging
Fluorescence Lifetime-Imaging
Microscopy (FLIM)
Förster Resonance Energy Transfer
(FRET)
Time-gated Raman / Laser Induced
Breakdown Spectroscopy (LIBS)
Time-resolved imaging & spectroscopy
Diffuse Optical Tomography (DOT)
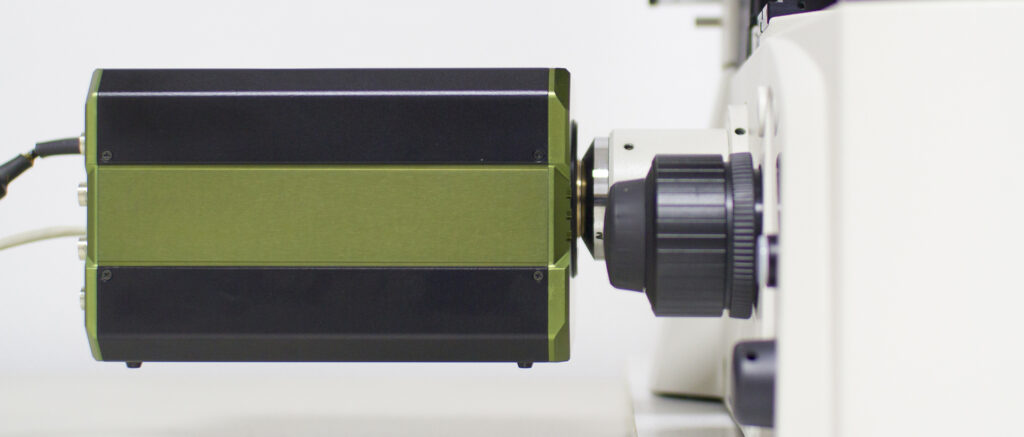
Features
Gated image intensifier
The TRiCAM is equipped with an integrated timing pulse generator and a gate-unit, generating gate pulses down to < 3 ns.
Low-light imaging
The TRiCAM has a built-in image intensifier that boosts the incoming light. This way, you can capture detailed images in the most challenging light conditions.
Fiber-optically coupled
Our experienced engineers couple the sensor to the image intensifier with a fiber-optic plate, resulting in a 10 – 20x more light efficient system. The fiber-optic plate is a solid piece of glass that consists of millions of parallel glass fibers sealed together. Each fiber acts as an independent light conductor that transfers the light from the image intensifier to the sensor.
Ultra-short gating
The image intensifier in the TRiCAM can be used as an ultra-fast electronic shutter. This technique is called gating and it can be done in a matter of nanoseconds.
Gating can eliminate motion blur when imaging fast-moving objects or highly dynamic processes. By varying the timing of the gate signal very precisely (tens of picosecond resolution), you can use gating to record a time-resolved light intensity profile.
Optimised for your application
The TRiCAM can be configured with a wide range of image intensifiers. Available image intensifiers cover the entire visual spectrum and the near infrared.
High-resolution sensor
The TRiCAM features a high-resolution CMOS sensor. It captures stunningly detailed images at 1920 x 1200 pixels.
160 fps
At up to 160 fps, the TRiCAM can record slow-motion footage, even in low-light conditions.
Global shutter
With its global shutter readout method, the sensor in the TRiCAM eliminates rolling shutter effects in your images.
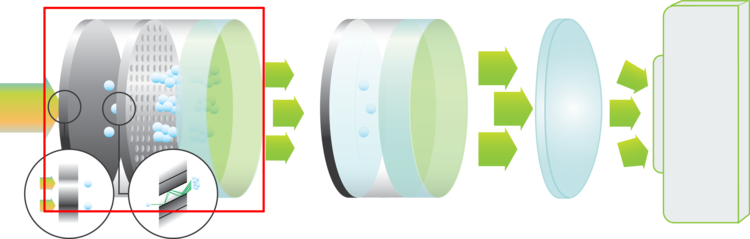
Image Intensifiers
An image intensifier boosts the intensity of the incoming light. By converting photons to electrons and back to photons, the light intensity can be increased significantly. Scroll down for more information about how this works.
Another feature of an image intensifier is that it can act as an ultra-fast shutter. The photocathode can be switched between on and off in a matter of nanoseconds.
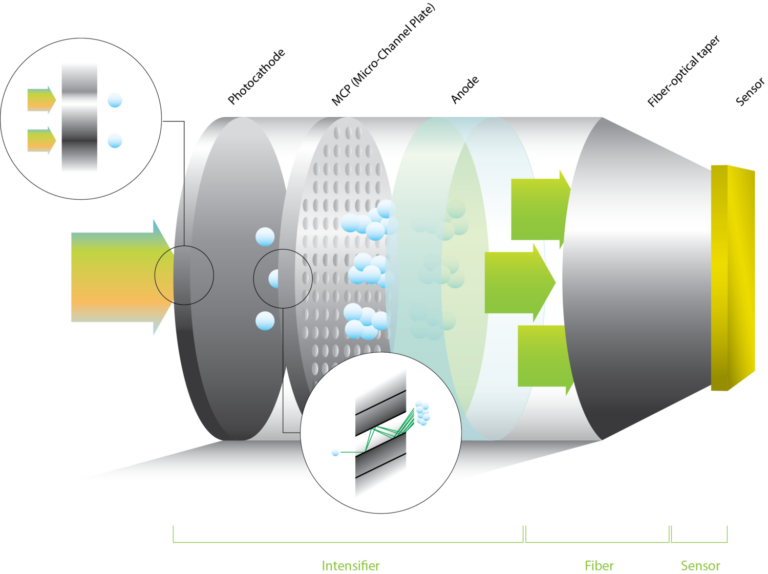
Low-light imaging
The TRiCAM has a built-in image intensifier that boosts the incoming light. This way, you can capture detailed images in the most challenging light conditions.
Our experienced engineers will help you pick the right image intensifier for your application.
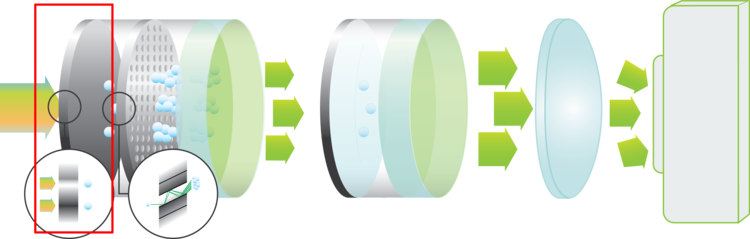
Photocathodes
The photocathode is the entrance of the image intensifier. This is where the incoming photons are converted to electrons. The quantum efficiency of the photocathode material specifies how efficient this conversion is for each wavelength.
Images

Specifications
IMAGE INTENSIFIER
Diameter: 18 mm
Minimum Gate Width: 40 ns (< 3 ns optional)
Maximum Repetition Frequency: 300 kHz
Trigger Input: TTL
SENSOR
Resolution: 1920 x 1200 pixels
Pixel Size: 5.86 µm
Frame Rate: 162 fps
Sensor Type: CMOS
Readout Method: Global Shutter
ADC: 10 bit and 12 bit
Request more information
Lambert Instruments BV
Leonard Springerlaan 19
9727 KB Groningen
The Netherlands
User Publications
Researchers around the world use the TRiCAM in their studies. Opposite is an overview of the most recent publications describing research that was done using a TRiCAM.
For a complete overview of applications, please visit our applications pages.

